The T3-4-Hypo trial
A Dutch national randomized placebo-controlled double-blind multicenter trial of LT4/LT3 combination therapy in patients with autoimmune hypothyroidism.

Introduction
Hypothyroidism is common, affecting 5% of the general population, for which levothyroxine (LT4) monotherapy is the standard treatment. Despite normalized serum thyroid hormone levels, 10-15% of LT4 (levothyroxine) treated patients have various persistent complaints, the most important of which is tiredness. This often leads to a poor quality of life, with withdrawal from society and work. The explanation for the persistent complaints could be that physiological T4/T3 ratios cannot be reached with LT4 monotherapy, as in a healthy individual T3 is not only derived from T4/T3 conversion but is also directly produced by the thyroid itself. Studies have reported contradicting results as to whether addition of liothyronine (LT4/LT3 combination therapy) in patients with persistent tiredness on LT4 monotherapy is effective or not.
This problem is a top prioritized knowledge gap on the national research agenda of the Dutch Society of Internal Medicine (NIV), as endorsed by both the Dutch Endocrine Society (NVE) and the Dutch thyroid disease patient organization SON (Schildklier Organisatie Nederland).
The aim
To investigate whether addition of liothyronine (LT4/LT3 combination therapy) in patients with persistent tiredness on LT4 (levothyroxine) monotherapy is effective or not in relieving tiredness.
Criteria
Criteria for
participation
Inclusion and exclusion criteria are:
In order to be eligible to participate in this study, an individual must meet all of the following criteria:
- Age 18 years or older;
- Overt or subclinical hypothyroidism*;
- LT4 monotherapy for at least 6 months;
- LT4 monotherapy dose of 75-225 microg, with at least a dose of 1.2 microg/kg;
- TSH levels within the assay-specific reference ranges for at least 3 months;
- Severe tiredness with a large negative impact on daily life for at least 6 months, with or without other persisting complaints, based on the patient’s own experience, without judgment of the treating physician;
- Sufficiently fluent in Dutch and able to read Dutch.
*Thyroid peroxidase (TPO) and/or thyroglobulin (Tg) antibody positivity is not a requirement as these have frequently not been determined. Instead, we ensure that we only include patients with autoimmune hypothyroidism by excluding other causes of hypothyroidism (see exclusion criteria).
An individual who meets any of the following criteria cannot participate in this study:
- Congenital hypothyroidism, hypothyroidism after (sub)acute thyroiditis, secondary (central) hypothyroidism;
- Thyroid surgery, radioactive iodine treatment, or head and/or neck radiotherapy;
- Use of thyroid interfering drugs (current/past use of amiodarone, immunotherapy, tyrosin kinase inhibitors, interferon, or lithium and current use of oral or iv corticosteroids or dopamine);
- Current psychiatric disease treated at a “gespecialiseerde GGZ instelling”*;
- Clinical diagnosis of dementia;
- Pregnancy, breastfeeding or wish to become pregnant within 2 years;
- Women of reproductive age not using adequate contraception, who are not sterilized and do not have a sterilized partner. Adequate contraceptives include the contraceptive pill, patch, injection, implant, intrauterine device or system, vaginal ring, diaphragm or cap, and condom;
- Functional or structural abnormal heart (e.g., cardiomyopathy or valve disease);
- Recent acute coronary syndrome or unstable angina pectoris (<4 weeks);
- Current/past atrial fibrillation;
- Current conduction disorder on ECG (i.e, QRS>100 ms or prolonged QTc (women≥460 ms and men≥450 ms));
- Frequent ventricular extrasystole (=doublet, trigeminy, bigeminy or (non-sustained) ventricular tachycardia) in the past or on current ECG.
- Other obvious medical explanation for tiredness (e.g. end-stage renal disease, anemia, COPD stage IV, cancer, etc.);
- Other obvious major life event explanation for tiredness (e.g., mourning, loss of job).
*Treatments of mild non-complex psychological/psychiatric complaints are performed in the “ basis GGZ”, e.g. consisting of conversations with a psychologist or psychotherapist, or via internet (e-health). “Gespecialiseerde GGZ” encompasses treatments of more severe psychological/psychiatric complaints.
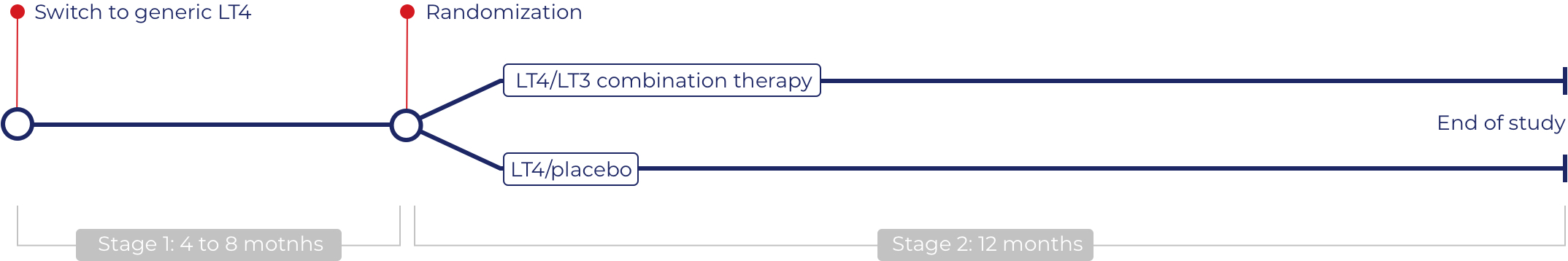
Study design
A Dutch national randomized placebo-controlled double-blind multicenter trial. Six hundred adult patients with autoimmune hypothyroidism will be included who despite biochemical euthyroidism on LT4 (levothyroxine) monotherapy have persistent tiredness with a negative impact on daily life.
Switch to generic LT4
In this run-in stage all patients switch to blinded generic LT4. This is because there are multiple LT4 preparations available with different pharmacokinetic properties, which would otherwise introduce substantial bias in the trial. Previous research has shown that 36% of patients need dose adjustments when switching to other LT4 preparations. Therefore, serum TSH levels are measured every 8 weeks, and medication dosages adjusted if needed, in order to obtain serum TSH levels within the assay-specific reference range. For trial feasibility, patients will be excluded and referred back to their referring physician when a normal TSH cannot be reached with a maximum of two dose adjustments. Once a normal TSH has been measured, the final run-in TSH measurement will be performed 8 weeks later. This is because we want to ensure that we only enrol patients with a stable (i.e. normal) TSH on a stable dose of generic LT4, as recent dose adjustments could otherwise impact the tiredness questionnaire scores at the start of the trial. Patients on generic LT4 will enter Stage 2 (randomized controlled trial) when they have a normal TSH, no ECG abnormalities, and indicate they have persistent tiredness. Patients not fulfilling these criteria will be excluded from this study and referred back to their referring physician. The expected duration of the run-in period will be 4-8 months, depending on the number of dose adjustments.
Randomized controlled trial (LT4/LT3 vs LT4/placebo)
Stage 2 includes a 1-year double-blind randomized placebo-controlled trial comparing LT4/LT3 with LT4/placebo treatment. At baseline, patients are randomized to either LT4/LT3 (16:1 ratio) or LT4/placebo treatment. Serum TSH levels are measured at every visit, and medication dosages adjusted if needed, with the goal to normalize serum TSH to levels within the assay-specific reference range. Visits will take place at baseline, and weeks 8, 16, 26, 39 and 52. The density of visits is higher at the beginning as this is the period when dose adjustments are particularly expected. At every visit, patients are asked to complete questionnaires on thyroid related complaints and quality of life (ThyPRO), general quality of life (EuroQoL-5D-5L and EuroQoL-5D-VAS), medical consumption (iMCQ) and productivity losses (iPCQ). At baseline and 52 weeks, additional blood will also be drawn to determine genetic variants, (thyroid hormone) metabolites and collect material for biobanking, as these data will be key to identify subgroups who are likely to respond to LT4/LT3 combination therapy.
Although no detrimental effects of these low physiological LT3 dosages are expected, and previous studies neither reported any detrimental effects, we will explore the effects of LT4/LT3 combination therapy on the most important thyroid hormone target organs (bone, cardiovascular, metabolic and brain). This will improve communication between medical professionals and patients, providing them with comprehensive information on the balance between the potential benefits and harms of combination therapy vs monotherapy and will enable shared-decision making that better addresses individual needs of patients.
For bone, serum markers will be determined at baseline and 52 weeks, and DXA scans will be performed at baseline and 52 weeks in a subgroup of 200 individuals. For cardiovascular and metabolic endpoints, blood pressure, pulse rate, weight, and waist circumference are measured at every visit. Fat percentages will be assessed on the beforementioned DXA scans. A repeat ECG will be performed at 52 weeks. To objectively explore effects on neurocognitive function, next to the subjective ThyPRO questionnaire scores, neurocognitive tests will be performed in a subgroup of 200 patients at baseline and 52 weeks.
The primary study outcome is the ThyPRO tiredness subscale score at 52 weeks.
Participating centers
Legenda
: DEXA-scan
: Neurocognitive test
| Center | Investigator | Location | Additional tests |
|---|---|---|---|
| Erasmus MC | Dr. M. Medici | Rotterdam | |
| Radboudumc | Dr. R.T. Netea-Maier | Nijmegen | |
| Rijnstate Ziekenhuis | Dr. S. Roerink | Arnhem | |
| UMC Groningen | Dr. W. Zandee | Groningen | |
| Maastricht UMC+ | Dr. B. Havekes | Maastricht | |
| UMC Utrecht | Drs. S.M. van der Leij | Utrecht | |
| Leids Universitair Medisch Centrum | Dr. M. Snel | Leiden | |
| Amsterdam UMC, locatie AMC | Dr. E. Bruinstroop | Amsterdam |
| Center | Investigator | Location | Additional test |
|---|---|---|---|
| Franciscus Vlietland ziekenhuis | Dr. M.E. Kevenaar | Schiedam | |
| Maasstad Ziekenhuis | Dr. C. van Noord | Rotterdam | |
| Máxima Medisch Centrum | Dr. P.C.M. Wouters-van Poppel | Veldhoven | |
| Admiraal De Ruyter Ziekenhuis | Dr. C.K.A. van den Berge | Goes | |
| Albert Schweitzer Ziekenhuis | Dr. R.M. Kiewiet-Kemper | Dordrecht | |
| Van Weel-Bethesda Ziekenhuis | Drs. E. van Schaik | Dirksland | |
| Zuyderland Medisch Centrum | Dr. R. Bianchi | Sittard-Geleen |
Contact
For any scientific or patient care related questions, please contact us by email t3hypotrial@erasmusmc.nl
